Let's Talk About PFAS
In an era of rapid technological and scientific advancement, we are surrounded by many substances that profoundly impact our daily lives, many of which remain unseen and unrecognized. Among these silent yet ubiquitous actors are a group of synthetic chemicals known as Per- and Polyfluorinated Substances (PFAS). Though they operate behind the scenes, their presence and influence are far-reaching. This article looks at PFAS, their chemical characteristics, and their applications across various industries.
What Are PFAS?
What Does PFAS Stand For?
The term PFAS stands for Per- and Polyfluorinated Substances. These are a group of man-made chemicals that have been in use since the mid-20th century. The "P" stands for per- and poly-, indicating that these substances contain fluorine atoms that are fully or partially substituted on a carbon chain. The "F" signifies Fluorinated, denoting the presence of the element fluorine in these substances. The "A" and "S" stand for Alkyl Substances, highlighting that these are organic compounds in which fluorine atoms replace hydrogen atoms on an alkyl chain.
The Broad Family of PFAS Chemicals
PFAS is not one single substance but a broad family of related chemicals. Over 4,700 different PFAS compounds have been identified to date. Despite the diversity within this group, all PFAS share a common characteristic: they contain at least one perfluoroalkyl moiety. In this molecule segment, carbon atoms are fully saturated with fluorine atoms. This perfluoroalkyl group is what imparts PFAS with its unique properties.
Well-Known PFAS: PFOA and PFOS
Among the vast family of PFAS, two compounds have become particularly well-known due to their widespread use and persistence in the environment: Perfluorooctanoic Acid (PFOA) and Perfluorooctanesulfonic Acid (PFOS). PFOA, commonly known as C8, was widely used in the production of Teflon, while PFOS was a key ingredient in Scotchgard, a fabric protector. These chemicals are notable for their extreme durability and resistance to degradation, earning them the nickname "forever chemicals."
As we delve deeper into PFAS, we will explore their unique chemical characteristics and role in various industries. In doing so, we hope to shed light on these invisible chemicals that have silently intertwined with our lives.
Chemical Characteristics of PFAS
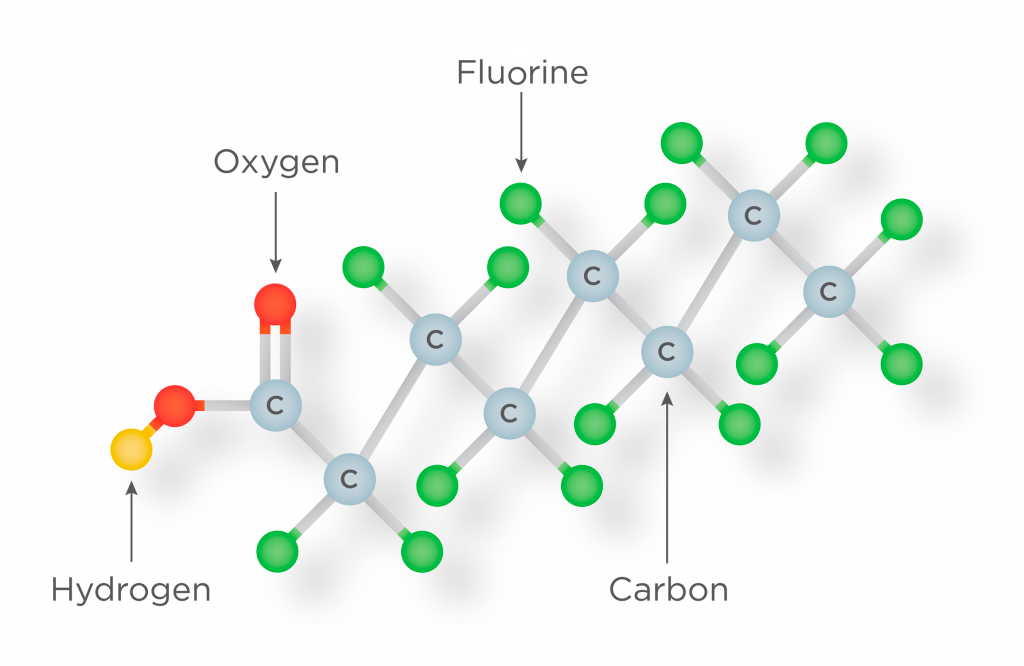
The Strength of the Fluorine-Carbon Bond
One of the defining features of PFAS is the carbon-fluorine bond. This bond is one of the strongest in organic chemistry, resulting in compounds that are incredibly stable and resistant to degradation. The carbon-fluorine bond's strength is due to fluorine's high electronegativity, which means it holds tightly to its shared electrons, creating a very strong and stable bond.
Thermal and Chemical Stability of PFAS
The robust carbon-fluorine bonds grant PFAS remarkable thermal and chemical stability. These compounds are resistant to heat, oil, water, and most acids and bases. This stability makes PFAS ideal for various industrial applications requiring durability and resistance to harsh conditions. However, this same stability also means that PFAS are not easily broken down in the environment or the human body, leading to their persistence and potential accumulation over time.
Hydrophobic and Lipophobic Properties of PFAS
PFAS are unique in that they are both hydrophobic (water-repelling) and lipophobic (fat-repelling). These twin properties arise from the fluorocarbon section of the molecule, which neither mixes well with water nor with oils. This makes PFAS excellent at repelling both substances, leading to their use in a wide range of products designed to resist stains, repel water, or reduce friction.
Long Environmental and Biological Half-Lives of PFAS
Due to their stability, PFAS have a long environmental and biological half-life. They do not easily degrade in the environment, leading to their accumulation in soil and water over time. In biological systems, PFAS can remain for years, resulting in bioaccumulation in organisms. This persistence in both the environment and living organisms has raised concerns about the potential health effects of PFAS.
As we proceed to the next section, we will further explore the unique nature of PFAS and why these properties have led to their widespread use across various industries.
Uniqueness and Industrial Use of PFAS
Uniqueness of PFAS
PFAS are unique in chemistry due to their remarkable stability, resistance to heat and chemicals, and dual hydrophobic and lipophobic nature. These properties stem from their defining fluorocarbon bonds, which grant them the durability and versatility that few other substances possess. Because of these features, PFAS has been aptly termed "forever chemicals," highlighting their persistence and widespread influence.
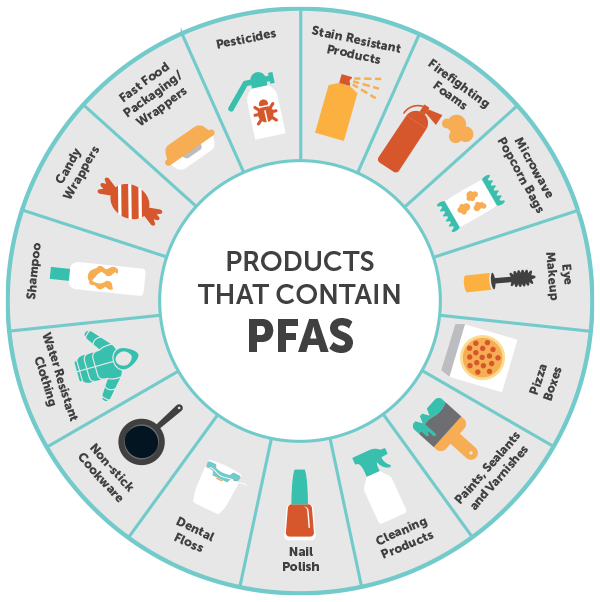
Industrial Use of PFAS
PFAS have found their way into diverse industries due to their unique characteristics. Below are some notable examples:
- Non-stick Cookware: The most well-known use of PFAS is in non-stick cookware, providing a smooth, heat-resistant, and easy-to-clean surface. The use of PFOA in the manufacture of Teflon is a classic example.
- Stain-Resistant Fabrics and Carpets: PFAS are used to treat fabrics and carpets to make them resistant to stains and water. Their hydrophobic and lipophobic properties make them excellent at repelling liquid spills.
- Firefighting Foams: PFAS, specifically PFOS, have been widely used in aqueous film-forming foams (AFFF), which are used to fight fuel fires. Their heat resistance and ability to spread easily on fuel surfaces make them effective in this role.
- Food Packaging: Some PFAS are used in food packaging materials to make them resistant to oil and grease. This keeps the packaging from becoming soggy or discoloured.
- Industrial Processes: PFAS are used in various industrial processes due to their heat resistance and ability to reduce friction. Examples include using cooling systems, hydraulic fluids, and electronic devices.
These are just a few examples of how PFAS have been integrated into our everyday lives. Their versatility and durability have made them invaluable across industries. Still, these same properties have also led to increasing concerns about their persistence in the environment and potential impacts on human health.
Conclusion
As we've explored in this article, Per- and Polyfluorinated Substances (PFAS) are a remarkable group of chemicals. Their unique combination of stability, resistance to heat and chemicals, and hydrophobic and lipophobic properties have made them invaluable across various industries. From non-stick cookware and stain-resistant fabrics to firefighting foams and food packaging, PFAS has become a ubiquitous part of our everyday lives.
However, these "forever chemicals" come with a double-edged sword. Their resistance to degradation means they persist in the environment and biological systems, leading to potential health and ecological concerns. As we continue to rely on these chemicals for their unique properties, we must also strive to understand and manage their impacts on our world.
Understanding PFAS is not just about recognizing their role in our lives and industries; it's also about appreciating the delicate balance between utilizing the beneficial properties of these substances and mitigating their potential risks. As we move forward, it's clear that PFAS will remain an important topic of research and discussion in chemistry, environmental science, and public health.
In the end, the story of PFAS is a testament to our ingenuity in manipulating the elements of nature to serve our needs. Still, it also serves as a reminder of the care we must take in managing the consequences of our inventions.

